
















Because the cryptocurrency trade strikes towards a thriving altcoin Season, common Shiba Inu staff member and advertising lead Lucie has proclaimed the season is the proper...


Kansas Metropolis Chiefs kicker Harrison Butker is dealing with backlash after his controversial commencement speech. Butker, 28, was the graduation speaker at Benedictine Faculty on Saturday,...


Inside minister says suspect charged with tried homicide is ‘lone wolf’ who had joined anti-government protests. Slovakia’s Prime Minister Robert Fico is steady however his situation...
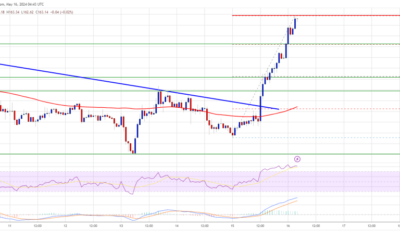

Solana began a contemporary improve above the $150 resistance. SOL worth is up almost 15% and may proceed to rise if it clears the $165 resistance....


Horizon: An American Saga — Chapter 1, the Western epic directed by and starring Kevin Costner, solely debuts on the Cannes Movie Competition 2024 on Sunday. However...


CNN — Slovakian police have charged a person, described as a politically motivated “lone wolf,” with the tried homicide of Prime Minister Robert Fico, who’s severely ailing in...


EA Sports activities revealed a launch date of the Faculty Soccer 25 online game on Thursday and a canopy picture that options Michigan operating again Donovan...


Be part of Our Telegram channel to remain updated on breaking information protection These days, early and common traders are actually into meme cash, and the...


Younger and the Stressed followers will see main developments within the lives of outstanding characters the week of Could 20-24,2024. Billy Abbott (Jason Thompson), away on a...


Tim Bontemps, ESPNMight 15, 2024, 09:24 PM ET BOSTON — The Celtics have been closely favored to beat the Cavaliers even earlier than Cleveland had three...